Paper published in Metal Construction, vol.14, no.2, Feb 1982, p73-79.
Part 2- Results and discussion
In Part 1, T G Gooch described a study of effects of nickel on the stress corrosion cracking behaviour of weld metals appropriate to structural and pipeline steels, in which tests were carried out using nickel-free and nickel containing electrodes, and electrodes without nickel but containing 1.6%Mn. The most important feature of the results, as discussed here, was that there was no indication that nickel has a major adverse effect on susceptibility to hydrogen induced SCC.
Results
SCC testing
Seawater studies
Results obtained are given in Tables 8 and 9 and summarised in Table 10. The 'threshold' stress intensity values in Table 10 are derived from the highest load supported by the sample without failure ( i.e. generally corresponding to the penultimate increment), rounded down to the nearest 50Nmm -3/2 . As noted above, no sample showed complete fracture, but only general yielding and bending to the limit stops of the machine. Such bending occurred either between load increments or during the application of increased load; in most cases, the stress intensity at which bending occurred was below the value derived from air tests ( Table 11).
Table 8 Weld metal SCC tests in seawater with cathodic protection
| Weld metal type | Initial stress intensity,
Nmm -3/2 | Final stress intensity,
Nmm -3/2 | Total test duration, hr | Comments |
|---|
| Static load | Final |
|---|
(a) At+20°C
7018 |
1480 |
3380 |
2040 |
4080 |
Bent on loading: no visible SCC |
| |
1710 |
2430 |
2020 |
2930 |
Failed between increments: 3mm SCC |
| |
1780 |
2530 |
2020 |
2920 |
Bent on loading: no visible SCC |
| 8018-C1 |
2380 |
3060 |
2090 |
2590 |
Failed between increments: c0.2mm SCC |
| |
2250 |
2250 |
4030 |
4030 |
Removed without failure |
| |
2460 |
3160 |
2060 |
2640 |
Bent on loading: no SCC |
| 1.6%Mn |
1620 |
3630 |
2110 |
4520 |
Bent on loading: no SCC |
| |
1690 |
3270 |
2020 |
3840 |
Bent on loading: c 1mm SCC |
(b) At+4°C
7018 |
860 |
1820 |
2110 |
3550 |
Removed without failure |
| |
2130 |
3400 |
552 |
770 |
Failed between increments: c 0.5mm SCC |
| |
2800 |
2800 |
0.1 |
0.1 |
No visible SCC |
| 8018-C1 |
950 |
2190 |
2040 |
3700 |
Removed without failure |
| |
1870 |
3020 |
2040 |
2660 |
Removed without failure |
| |
2240 |
3020 |
240 |
650 |
Failed between increments: c 1mm SCC |
| 1.6%Mn |
900 |
1900 |
2100 |
3800 |
Removed without failure |
| |
1720 |
2740 |
350 |
910 |
Failed between increments : no visible SCC |
| |
2050 |
2706 |
550 |
720 |
Failed between increments: c 2mm SCC |
Table 9 HAZ SCC tests in seawater with cathodic protection at +20°C
| Sample type | Initial stress intensity, Nmm -3/2 | Final stress intensity, Nmm -3/2 | Total test duration, hr | Comments |
|---|
| Static load | Final |
|---|
| Hard HAZ |
670 |
1370 |
2060 |
3300 |
Removed without failure |
| |
840 |
1700 |
2060 |
3500 |
Removed without failure |
| Soft HAZ |
1700 |
1700 |
4300 |
4300 |
Removed without failure |
| |
1290 |
2300 |
600 |
820 |
Bent between increments: c 0.1mm SCC |
| |
2010 |
2300 |
600 |
740 |
Bent between increments: c 2mm SCC |
Table 10 Summary of pre-cracked sample SCC tests in seawater
| Sample type | Approximate threshold stress intensity, Nmm -3/2 |
|---|
Seawater/cathodic protection/
+20°C | Seawater/cathodic protection/
+4°C | Seawater/100ppm H 2 S |
|---|
| 7018 |
2350 |
2800 |
3100 |
| 8018-C1 |
2900 |
2950 |
2950 |
| 1.6%Mn |
3150 |
2650 |
2750 |
| Hard HAZ |
>1700 |
nd |
<2360 |
| Soft HAZ |
2100 |
nd |
nd |
| nd =not determined |
Table 11 Fracture toughness tests in air
| Sample type | Stress intensity at maximum load*
K c , Nmm -3/2 |
|---|
| 7018 weld metal |
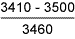 |
| 8018-C1 weld metal |
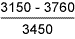 |
| 1.6%Mn weld metal |
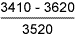 |
| Hard HAZ |
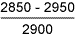 |
| Soft HAZ |
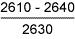 |
| *(i) Triplicate samples tested |
| (ii) All samples displayed stable maximum load behaviour with plastic strain at the crack tip |
| (iii) K c calculated assuming negligible tearing prior to attainment of maximum load |
|
From the failure behaviour, it was evident that all specimens showed high resistance to SCC [23], as further indicated by the fact that there was no indication of crack extension at applied stress intensity values below about 2000Nmm -3/2. In terms of stress intensity to cause 'failure', the results obtained indicate that the 8018-C1 consumable was superior to the 7018, and to HAZ samples at +20°C, although the 'threshold' stress intensity was highest for the 1.6%Mn deposit. At +4°C, the 1.6%Mn deposit appeared the most susceptible, with the 7018 having highest SCC resistance. However, particularly bearing in mind the macroscopically ductile failure mode observed, it is unlikely that the differences are of great practical significance. It will be recognised from the stress intensity data in Tables 9-11 that sample dimensions were too small to give plane strain crack tip conditions. [29] Thus, calculation of applied stress intensity levels should not be regarded as precise, but it is considered unlikely that such deviation from plane strain conditions significantly affects assessment of relative material behaviour.
NACE studies
Results of samples taken transverse to welded joints are given in Fig.3, and Table 12. All specimens showed fracture in parent material away from the weld area. Sectioning revealed that cracking had occurred in parent material and HAZs ( Fig.4), predominantly in association with inclusions as is the case with 'hydrogen pressure cracking' (HPC) [17,18] , although some branching was noted in the HAZ, particularly close to the fusion boundary. No weld metal cracking was observed. It will be noted from Table 12 that, although a threshold stress for parent material was not rigorously established, it was certainly substantially below the parent material yield stress.
Table 12 Summary of NACE test threshold stress levels
| Sample type | Mean yield stress, N/mm 2 | Threshold stress, N/mm 2 |
|---|
| 7018 weld metal |
490 |
<280 |
| 8018-C1 weld metal |
585 |
345 |
| 1.6%Mn weld metal |
530 |
345 |
| Parent material |
350 |
200 |
Results from all weld metal tensile examples are also shown in Fig.3 and Table 12. In terms of the 'threshold' stress to cause failure within a maximum exposure period of 720hr, highest susceptibility was shown by the 7018 consumable, with there being no significant difference between the 8018-C 1and 1.6 % Mn consumables.
Low H2S studies
Results obtained are given in Table 13. Sample behaviour was generally similar to that in the plain seawater studies considered above, with bending taking place rather than complete fracture. There was no consistent effect of H 2 S on 'threshold' stress intensity level, although SCC to a greater total crack depth was found, but again the stress intensity levels at failure were high. It will be noted that in three cases, crackingdeveloped in the HAZ ( Fig.5). A single test was carried out on a K preparation 'hard' HAZ sample. The result is included in Table 13 for comparison, and indicates higher SCC sensitivity in the HAZ than in the weld metals studied. The results are summarised in Table 10.
Hydrogen pick-up behaviour
Results of the hydrogen analyses are shown in Table 14. Pick-up in seawater with cathodic protection was negligible at both +20°C and +4°C for all samples. This was essentially the case also in seawater with 100ppm H 2 S, although a diffusible hydrogen level of 0.18 ml/l00g was found for 7018 weld metal after 2650hr, suggesting that the presence of H 2 S can increase hydrogen pick-up.
Table 14 Results of hydrogen determinations
| Environment | Exposure time, hr | Sample type | Hydrogen level, ml/100g |
|---|
| Diffusible | Residual | Total |
|---|
| Sea water with cathodic protection +20°C |
0 |
7018 |
0.00 |
0.05 |
0.05 |
| |
|
8018-C1 |
0.00 |
0.04 |
0.04 |
| |
|
1.6%Mn |
0.00 |
0.04 |
0.04 |
| |
100 |
7018 |
0.00 |
0.05 |
0.05 |
| |
|
8018-C1 |
0.00 |
0.05 |
0.05 |
| |
|
1.6%Mn |
0.00 |
0.05 |
0.05 |
| |
1000 |
7018 |
0.00 |
0.05 |
0.05 |
| |
|
8018-C1 |
0.00 |
0.05 |
0.05 |
| |
|
1.6%Mn |
0.00 |
0.05 |
0.05 |
| |
4000 |
7018 |
0.00 |
0.05 |
0.05 |
| |
|
8018-C1 |
0.00 |
0.06 |
0.06 |
| |
|
1.6%Mn |
0.00 |
0.08 |
0.08 |
| Sea water with cathodic protection +4°C |
100 |
7018 |
0.00 |
0.16 |
0.16 |
| |
|
8018-C1 |
0.00 |
0.13 |
0.13 |
| |
|
1.6%Mn |
0.00 |
0.04 |
0.04 |
| |
1000 |
7018 |
0.00 |
0.03 |
0.03 |
| |
|
8018-C1 |
0.00 |
0.02 |
0.02 |
| |
|
1.6%Mn |
0.00 |
0.05 |
0.05 |
| |
3400 |
7018 |
0.00 |
0.04 |
0.04 |
| |
|
8018-C1 |
0.00 |
0.02 |
0.02 |
| |
|
1.6%Mn |
0.00 |
0.03 |
0.03 |
| NACE |
0 |
Parent plate |
0.00 |
0.06 |
0.06 |
| |
100 |
Parent plate |
8.07 |
0.24 |
8.31 |
| |
|
7018 |
0.94 |
0.14 |
1.08 |
| |
|
8018-C1 |
2.60 |
0.11 |
2.71 |
| |
|
1.6%Mn |
1.54 |
0.16 |
1.70 |
| |
525 |
Parent plate |
7.21 |
0.59 |
7.80 |
| |
|
7018 |
2.73 |
0.31 |
3.04 |
| |
|
8018-C1 |
0.39 |
0.11 |
0.50 |
| |
|
1.6%Mn |
0.75 |
0.14 |
0.89 |
| Sea water with 100ppm H 2 S |
350 |
8018-C1 |
0.04 |
0.04 |
0.08 |
| |
|
1.6%Mn |
0.05 |
0.03 |
0.08 |
| |
2650 |
7018 |
0.18 |
0.03 |
0.21 |
| |
|
8018-C1 |
0.00 |
0.02 |
0.02 |
In contrast, appreciable hydrogen was picked up under NACE conditions, but it will be noted that most rapid ingress was found with parent material rather than weld metal.
Overall, some variation in pick-up rate is evident, but the results do not indicate nickel to have a consistent adverse effect in respect of hydrogen ingress.
Metallographic observations
Representative weld metal and HAZ microstructures are shown in Fig.6-9. As deposited, the 8018-C 1 consumable gave a substantial proportion of acicular ferrite with some grain boundary ferrite and ferrite with aligned second phase in low dilution runs, with the development of essentiallyequiaxed ferrite in reheated regions ( Fig.6). A somewhat coarser structure was apparent in the high dilution root runs both as-deposited and following reheating. In both high and low dilution regions, the 7018 electrodes gave a very non-uniform microstructure,with grain boundary ferrite, lamellar ferrite containing aligned second phase, ferrite-carbide aggregate and acicular ferrite, the last named being somewhat coarser than in 8018-C1 deposits ( Fig.7). As deposited runs using the 1.6%Mn consumable showed a high acicular ferrite content, although the individual units appeared longer than with the 8018-C1 weld metal ( Fig.8). High dilution regions were comparable with those obtained with the 8018-C1 electrodes.
The HAZ microstructures showed the expected range of martensitic and softer transformation products ( Fig.9).
Assessed visually, there appeared little difference in HAZ structure between the 'hard' and 'soft' welds.
Fractographic examination was hindered by the development of chalking within the crack in cathodically protected samples and by corrosion in specimens exposed to the H 2 S media. It was therefore difficult to determine the fracture mechanism unambiguously. Where this could be defined in the cathodically protected samples (in which it will be noted, crack growth took place onlyto a limited extent), failure was by microvoid coalescence (MVC). This appeared to be the case also with samples fractured under NACE and low H 2 S conditions, although the parent material NACE fractures displayed the stepped morphology characteristic of 'HPC'. [17,18]
Discussion
Overall
The salient feature of all the tests carried out under the different environmental conditions employed is that there is no indication that nickel has a major adverse effect on susceptibility to hydrogen induced SCC. This is true both for direct SCC tests and for studies on hydrogen pick-up behaviour. As pointed out above, the tests were carried out on consumables of controlled and constant composition, apart from those elements varying deliberately, and involved identical welding conditions and parent material. A direct parallel may be drawn between the present findings on weld metal and those of Snape [7] on low alloy parent steel in which only the nickel content was varied: in neither case is any detrimental effect of nickel apparent, consistent with other results obtained by Schmid [15] and by Keller and Cameron. [11] Thus, at least up to the level of nickel considered ( c 2.2%), there seems no reason to debar nickel per se from weld metal employed for H 2 S containing media.
In general terms, it will be accepted that the presence of nickel may tend to increase deposit hardness and strength and hence the risk of SCC, but it will be noted in the present work that the yield strength of the nickel containing consumable tested was above that of the 7018 electrode, and yet no adverse effect of nickel was identified. Hence, it may be that the hardening effect of nickel in carbon-manganese type weld metal will not be significant,although special consideration may be necessary should a particular weld have low heat input and attendant high cooling rate, with the possible formation of significantly harder transformed microstructures than presently observed.
Effect of environment
The ready failure observed under NACE conditions as compared with the seawater studies highlights the adverse effect of H 2 S on cracking risk. However, from both the SCC testing of pre-cracked samples and study of hydrogen pick-up rate, it seems that H 2 S levels of about 100ppm have only a limited influence on risk of weld metal SCC, although some caution is necessary since Hudgins et al [28] have indicated that extremely long test durations (>lyr) may be required to obtain a definitive assessment of behaviour at low H 2 S levels.
At present there is debate as to the H 2 S activity at which an environment should be considered sour, and hardness control to avoid SCC is required. It can be assumed that susceptibility will diminish progressively with reduction in H 2 S level, and the present results are encouraging in indicating that development of low H 2 S levels from bacterial action in marine service is unlikely to promote a greatly increased risk of SCC. This conclusion is consistent with work by Kihara et al [13] on structural steel weld metal, involving both laboratory and in-plant tests.
Material behaviour
It is now well established [17,18,30,31] that structural and pipeline steels can suffer cracking associated with hydrogen pick-up and development of an internal hydrogen pressure at inclusions. The present parent Grade 50D material exhibited this effect, with the result that failure under tensile loading in NACE conditions occurred at appreciably lower stresses than was the case for weld metal. This implies that in many cases it will be parent material properties which are limiting onstructural integrity, rather than weld metal or HAZ SCC susceptibility, and this should be clearly recognised. More work is required to define the probability of service failure by hydrogen pressure cracking, particularly under theaction of an applied stress, [31] but this failure mechanism must be considered when welded structural steel fabrications are employed for sour duties.
Further, it will be noted that regardless of parent material behaviour, some propensity was observed for cracking in the presence of H 2 S to develop in the HAZ rather than weld metal. Cracking was found in the HAZ in the transverse weld NACE tensile tests, but not in weld metal, while in some pre-cracked weld metal samples tested with 100ppm H 2 S, cracking deviated into the HAZ, with propagation in regions of higher hardness than the adjacent weld metal. The pre-cracked HAZ sample tested with 100ppm H 2 S also indicates a threshold stress intensity below the weld metal results. Hence, given a parent steel resistant to HPC, HAZ SCC resistance may well be lower than that of weld metal, whether or not the weld metal contains nickel.
Overall, the sample failure behaviour observed is consistent with that expected from consideration of the microstructures and hardness levels observed. Two points can be made. First, the risk of SCC in marine conditions at both+20°C and +4°C is evidently low, as illustrated by the essentially plastic failure behaviour, and, at the hardness levels involved, this is consistent with other work on structural steel weldments. [23,32,33] Second, SCC was found at stress levels below the yield stress in all weld metals under NACE conditions, with hardness levels below the Rockwell C22 (HV243) criterion [2] commonly applied to avoid H 2 S induced failure. It is recognised that weld metal can have increased susceptibility to cracking [34] and weld metal deposited using automatic processes may be limited to HB200 (HV205). Manual metal arc weld metals are exempt from this requirement, but on the basis of this and other work [15] , this exemption should be questioned. It is evident that further study is necessary both to define limiting hardness levels for weld metal types deposited using different processes and to examine the risk of service failure ata given hardness level, as opposed to failure under the severe NACE test conditions.
It will be noted that the 'threshold' stress intensity values in Table 10 are below the air toughness data in Table 11. It is known [35,36] that time dependent ductile tearing can take place in pre-cracked samples under constant load conditions in air, such tearing developing at applied loads below the maximum found in a conventional rising load toughness test.Bearing in mind the essentially plastic failure behaviour of the present pre-cracked SCC samples, it is probable that this phenomenon has contributed to some extent to the cracking found. However, it is unlikely that all cracking stems from this cause, both in view of the extent of SCC (up to 8mm SCC propagation), and since the 'threshold' levels in Table 10 are appreciably below the respective toughness data in Table 11; certainly the SCC 'thresholds' are less than 95% of the air tests, this proportion having been suggested [35] as a practical (although not necessarily absolute) limit for time dependent crack growth.
Summary and conclusions
Work has been carried out to examine the effect of nickel on the SCC behaviour of as-deposited ferritic steel weld metals appropriate to structural and pipeline applications. Tests were carried out on nickel-free (AWS E7018) and.nickel containing (AWS E8018-C1) electrodes, and electrodes without nickel but alloyed with 1.6%Mn to give strength levels similar to the 8018-C 1 type. Stress corrosion testing was carried out in the laboratory in simulated seawater with cathodic protection, in H 2 S saturated NACE conditions and in simulated seawater with c. 100ppm H 2 S. In addition, hydrogen pick-up behaviour was studied. It was concluded that:
- No effect of nickel on stress corrosion behaviour was found, at least under the welding conditions and nickel contents (0-2.2%Ni) employed.
- Nickel had no significant effect on weld metal SCC resistance in simulated seawater with cathodic protection or when containing c. 100ppm H 2 S. In the latter environment, some propensity for preferential HAZ crack propagation was observed.
- Nickel had no adverse effect on weld metal SCC resistance under NACE conditions. The lowest threshold stress observed was for the nickel-free 7018 weld metal. 4 Under NACE conditions, parent material failure by hydrogen pressure cracking was noted at applied stress levels below the threshold for weld metal cracking. Thus in many situations, weld metal behaviour will not necessarily be limiting on weldment performance, and it will be essential to test all regions of a welded joint (see for example conclusion 2).
- Hydrogen pick-up rate in the various media was not consistently influenced by nickel. Highest pick-up rate was found in parent material under NACE conditions.
Acknowledgements
The author thanks his colleagues at The Welding Institute for their advice and discussion regarding the above investigation. Particular acknowledgement is made to Messrs B J Ginn, G Regelous and B Kersey for their experimental assistance.
Grateful acknowledgement of support is made to the Engineering Materials Requirements Board of the Department of Industry and to:
BOC Murex
British Petroleum Co Ltd
British National Oil Corporation Det Norske Veritas
ESAB AB
Kockums AB
Norsk Hydro A/S
Shell International Petroleum Maatschappji
Statoil
References
- Uhlig H H: 'Corrosion and corrosion control'. Publ. John Wiley and Sons Inc, New York, 1963.
- NACE Standard, MR-01-75 (1980 revision).
- Treseder R S and Swanson T M: Corrosion 1968 24 (2) 31-37.
- NACE Standard, TM-01-77.
- Burns D S: Mat Perf 1976 15 (1) 21-28.
- Snape E: Corrosion 1967 23 (6) 154-172.
- Idem. Ibid 1968 24 (9) 261-282.
- Snape E: vide ref. 3.
- Kowaka M and Nagata S: Proc. 'Twelfth Japan Congress on Materials Research'. Soc. Mat. Sc. 1969, 141-145.
- Fraser J P and Eldredge G G: Corrosion 1958 14 (11) 44-50
- Keller H F and Cameron J A: Proc. 'Corrosion 74', NACE, Chicago, 1974.
- Watanabe M and Mukai Y: Osaka University Technical Reports, 1964 14 (602)
- Kihara M et al: Proc. 'Seventh World Petroleum Congress'. 1967, 235-260.
- Nakayama H et al: Welding World 1968 20 (11) 509-515.
- Schmid G C: Proc. 'Corrosion 79'. NACE, Atlanta, Georgia, 1979.
- Okada H et al: Proc Conf 'Stress corrosion and hydrogen embrittlement of iron-base alloys', Firminy, France, 1973, publ NACE, 1977.
- Moore E M and Warga J J: Mat Perf 1976 15 (6) 17-23.
- Inagaki H et al: Trans ISIJ 1978 18 149-156.
- Yukawa K et al: Corrosion Eng 1966 15 (10) 445-451.
- Dunlop A K: Corrosion 1978 34 (3) 88-96.
- Phelps E H and Loginow A W: Corrosion 1960 16 (7) 325t-335t.
- Barth C F, Steigerwald E A and Troiano A R: Corrosion 1969 25 (9) 353-358.
- Gooch T G: PhD Thesis, University of London, 1975.
- Judy R W Jr. and Goode R J: NRL Report, No. 6988, Nov. 1969.
- Kane R, Watkins M and Greer J B: Proc. 'Corrosion 76', NACE Houston, 1976.
- Efird K D and Lee T S: Corrosion 1979 35 (2) 79-83.
- Biefer G J and Garrison J G: Agard Conf Proc: No. 98, Paper 11, NATO, Brussels, 1971.
- Hudgins C M et al: Corrosion 1966 22 (8) 238-251.
- ASTM STP 381, 1965.
- Robinson J L: Welding Institute Research Bulletin 1977 18 (5) 121-126.
- Iino M: Met TransA 1978 (9a, Nov) 1581-1590.
- McDonald R D: Weld Res Abroad 1972 XVIII, June-July 23-34.
- Townsend H E: ASTM STP 518, 1972, 155-166.
- NACE Standard RP-04-72.
- Towers O L and Garwood S J: Proc 'Third Colloquium on Fracture,' London, Sept. 1980, 57-68.
- Tsuru S and Garwood S J: Proc Third Int Conf on 'Mechanical Behaviour of Materials,' Cambridge, Aug. 1979, 519-528.