Wed, 08 April, 2020
UK industry has rapidly stepped up to provide the NHS with products needed in the fight against COVID-19. TWI has been supporting this effort in a number of products, including ventilators, hand sanitiser and facemasks. The current crisis has seen some unprecedented changes, including a re-evaluation of the balance between conventional testing and validation processes vs. the consequences of having no safety product available. This has resulted in a code exemption for applicable products (dramatically reducing the level of testing required). However, it remains critical that products made available are both safe and effective.
Facemasks present a particular challenge. As a low cost, high volume disposable item, they require simple but effective design and compatibility with high volume manufacturing techniques. The challenge comes with the need to expand production beyond existing facilities; this either means changing design and materials to suit the available equipment set, or bringing in new equipment with a consequent need for a very rapid ramp-up in understanding of unfamiliar processes.
Industry is running with both approaches in parallel and TWI is providing technical support to enable this effort. Conventional manufacturing of facemasks uses solid-state welding of polymers, a technology where Principal Project Leader Scott Andrews has been providing training to industry for over 25 years. Scott is now using this expertise to help industry ramp up its level of expertise in preparation for deployment.
The alternative approach is to develop innovative new products which are designed to be rapidly scaled up for manufacture on existing equipment sets, often outside of conventional medical device manufacturing. This approach presents a number of challenges. Firstly, the design has to be validated; Tyler London, who leads TWI’s modelling and simulation group, has been working with Respolab Ltd on Teesside to validate their design, which was produced in only six days. This process, which would normally take many months, has been dramatically reduced to only a few weeks through a combination of advanced flow modelling using simulation software and targeted validation through physical testing at TWI’s laboratories in Cambridge. Flow simulations for inhale/exhale flow rates are being modelled and TWI is using computational fluid dynamics and analysis to show that code exemption can be justified on the basis of numerical results. This will eliminate the delay in transition to mass production (provided the code compliant tests are undertaken in due course), reducing the total time from conception to production to under a month.
Parallel activity has seen designs which incorporate laser cut components. Ali Khan of TWI’s laser group has been coordinating links between the medical design companies and facilities for laser cutting polymers, drawing on TWI’s long-standing links with manufacturing industry, including its membership of the Association of Industrial Laser Users.
The fight against COVID-19 will require a national effort and TWI is, and will continue to be, at the forefront in providing expertise to enable rapid and effective action to combat this crisis.
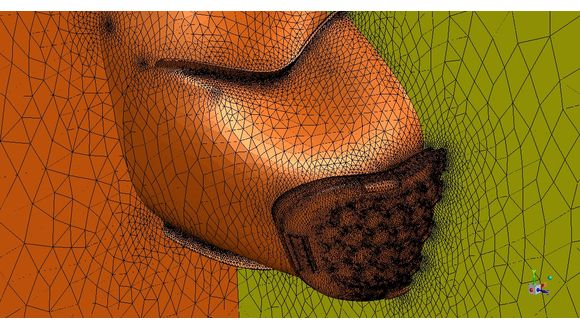
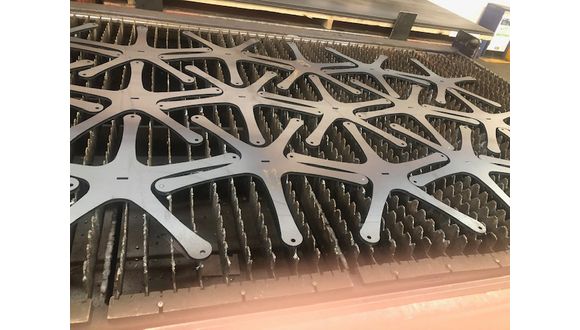 Image courtesy of Professor John Power of Laser Expertise Ltd
Image courtesy of Professor John Power of Laser Expertise Ltd