Stuart Bond
MICorr, NACE Senior Corrosion Technologist, TWI
Prepared for: Prevention and Management of Marine Corrosion, London, 2-3 April 2003. Lloyds List Events.
Summary
Welding influences material properties and must be considered in terms of design and initial fabrication and any subsequent repair during the life of a vessel. Additionally, new fabrication processes are being developed such as laser and laser hybrid welding, friction stir welding of aluminium alloys and improved wet underwater repair, necessitating understanding of the corrosion performance of such new weldments which may be used in the marine shipping industry.
The issues influencing the corrosion performance of welded carbon and low alloy steels, corrosion resistant alloys and aluminium alloys are presented. Possible issues related to wet underwater repair welds for permanent solutions are highlighted. Coating of weldments can be more difficult than parent material due to the weld profile and accessibility, whilst fatigue at welds may cause coating failure. Future applications of improved coatings based on thermally sprayed aluminium to protect carbon steels and corrosion resistant alloys in the marine environment is discussed. Recommendations of areas for further assessment in terms of long term corrosion performance of welds and corrosion fatigue in sour oil storage tanks are provided.
Introduction
Welding influences material properties and must be considered in terms of design and initial fabrication and any subsequent repair during the life of a vessel. Additionally, new fabrication processes are being developed such as laser and laser hybrid welding, friction stir welding of aluminium alloys and improved wet underwater repair, necessitating understanding of the corrosion performance of such new weldments which may be used in the marine shipping industry. Comment is made regarding the availability of information related to corrosion performance of such weldments. These areas are discussed in terms of the present knowledge, or experience in other sectors and the potential areas for future development to enhance corrosion mitigation measures available in the management of marine corrosion in the shipping industry.
Generally, if the corrosion mitigation measures are correctly applied and good inspection and maintenance adhered to, then welds should not pose any increased risk of corrosion failure, but owners must understand the potential issues associated with welds and likelihood of corrosion e.g. coating failure at welds. In optimising maintenance strategies, it is essential therefore that consideration be given to the likelihood of corrosion rates at weldments being higher than the anticipated rate for unwelded parent material.
Corrosion mitigation via barrier coatings remains the largest corrosion-related cost to all industrial sectors worldwide, with organic coatings accounting for 88% (US$107billion) of the annual USA expenditure on corrosion control methods and services, and metallic coatings accounting for US$1.4billion per annum in 2002. [1,2] The estimated annual cost of corrosion for the US marine shipping industry in 2002 was US$2.7billion, with some US$1.1billion expended on new build, US$0.8billion on maintenance and repair, and US$0.8billion due to downtime. Improved coatings designed to last the lifetime of a vessel offer opportunities for reduced whole life costs. Developments in the oil $ gas sector using thermally sprayed aluminium may be applicable to the shipbuilding and shipping sector to produce coatings with improved protection characteristics and life to first maintenance.
Corrosion resistant alloys (CRAs) are usually selected based on their inherent resistance to attack in marine exposure or to avoid additional operational costs associated with corrosion mitigation of steel. Additionally, and probably more frequently, CRAs may be chosen for the containment of process conditions related to oil and gas production facilities, transportation of chemicals etc but may be subject to external exposure to the marine environment. When such exposure is combined with residual stress and high operating temperatures, it can lead to localised corrosion and environmentally assisted cracking at weldments.
Welded carbon steel and low alloy steels in marine environments
Introductory comments
Whilst welding can have significant influence upon the corrosion performance of materials, for carbon steels exposed to marine environments, it is recognised that best practice in mitigation of general corrosion through coatings, cathodic protection or inhibition will usually also avoid any enhanced attack at weldments. However, where these measures fail, or are inadequate, the corrosion behaviour of a weldment can be significantly different to that of unwelded parent material.
The use of higher strength steels for offshore and shipping applications has been studied for over four decades, primarily with concern for mechanical performance in cold waters and also with regard to the issue of preferential weldment corrosion which is discussed later. Increasing the strength of the structural steel offers the benefit of reduced steel weight/physical dimensions and hence allows increased vessel capacity. [3] Higher strength steels such as those produced by TMCP (thermo-mechanically controlled processing) or low alloy steels can be used. TMCP processing allows the derivation of higher strength compared with normalised product fromthe identical steel chemistry, through the deformation and straining occurring during manufacture of the sheet. [4] Conversely, if a TMCP material of identical strength to a normalised product were selected, the former would have a leaner composition. TMCP steels manufactured with accelerated cooling are weldable, provided care is taken touse a moderately low heat input (<2.5kJ/mm for 15mm plate) such that the cooling rate is high and therefore the fine grain sizing leading to the desired properties developed during manufacture can be retained. TMCP steels manufactured without accelerated cooling are more readily weldable as there is less concern regarding heat affected zone (HAZ) softening.
The major issue associated with the use of higher strength steels, such as TMCP grades, in the shipping industry relates to the fact that design allows the material to be thinner. Consequently, when the same maintenance strategies have been applied as for carbon steels (where a certain amount of corrosion loss of wall section can be tolerated), this can lead to through-wall penetration of the thinner, higher strength material. It is essential, therefore, that full consideration be given to the likelihood of corrosion, the corrosion rate and consequences of through-wall failure when considering corrosion mitigation and selection of coating system. Higher strength steels also lead to some concerns regarding the loss of rigidity of the vessel structure [2] and the apparent increased corrosion rates reported for such steels may be ascribed to the spalling of corrosion products due to the flexing movement of the substrate. This spalling removes the overlying iron oxides, etc, and hence eliminates the diffusion rate controlling step for oxygen access to the region, allowing corrosion to occur on freshly exposed, active material.
Earlier developments on higher strength steels focused upon improved strength through Mn additions and retention of HAZ toughness via the addition of alloying elements such as Nb, Ti and V etc. [5] Such steels, in the unwelded condition, have a corrosion performance for immersed conditions very similar to that for carbon steel. [3] In addition, weldments in hardenable higher strength grades of steel can be susceptible to preferential corrosion when immersed, so that in regions where loss of coating can occur through abrasion, impact or wear, additional effort is required to ensure preferential corrosion will not take place.
Corrosion and corrosion fatigue in seawater and splash zone
The most common corrosion issue associated with carbon steels in shipping is the external corrosion of the hull, and corrosion rate will be dependent upon conditions of operation. In general, no corrosion allowance is made in shipping design, [2] primarily due to expectation of mitigation via coatings and cathodic protection (CP). The average corrosion rate for parent carbon steel under conditions of total immersion in seawater is estimated as 0.1mm per annum, [2,3,6] and 0.4-0.5mm per annum in splash zones [6] due to higher oxygen availability. Initial rates in short-term exposure will be higher and increased temperature can have a significant effect. The velocity of the water will have an impact and a flow rate of 1.5m.s -1 will increase by three times the corrosion rate in quiescent conditions. [6] However, as discussed later, the corrosion rate of an immersed weldment can be considerably higher than parent material, and caution is needed in estimating the likely corrosion that will occur in the event of coating failure ifCP is inadequate or the weld is in the splash zone.
Corrosion fatigue crack propagation for TMCP steels in seawater was studied by Nakano et al [7] regarding comparison of TMCP and normalised steel grades in artificial seawater at free corrosion potential and with cathodic protection, but significantly welds were not assessed. They concluded that there was no difference in the crack propagation rate between these steels in seawater, or in the acceleration measured under cathodic protection. Welds are invariably the point of concern for fatigue and corrosion fatigue. TWI has worked on the subject of corrosion fatigue of welds under CP and demonstrated that crack propagation rates can be higher in hardened weld area microstructures than the parent material. [8,9]
Atmospheric corrosion of welded steels and degradation in ullage spaces
Atmospheric corrosion of steel in marine environments can range from 0.03mm per annum in temperate zones to 0.07mm per annum in tropical coastal locations, [10] attributed to increased temperature and humidity. Atmospheric corrosion rates for shipping would be expected to be faster, necessitating coatings that can provide long term protection and have good resistance to undercutting. Corrosion in occluded areas and ullage spaces, when oxygen is present, can be faster than on external surfaces especially where the tanks are hot or ambient temperatures are high. Typically, based on the Arrhenius relationship for the rate of reaction, a 10°C increase in temperature can double the corrosion rate, thus providing particular concern for shipping operating in tropical and equatorial waters, or carrying hot oil cargo. Acidic condensate from oil cargo can also accelerate corrosion in tank ullage. [2]
Whilst preferential weldment corrosion is not believed to be a concern in marine atmospheres or ullage spaces due to the presence of only thin aqueous films that limit the area ratio of anode to cathode, weldments can be areas of corrosion susceptibility in these conditions due to issues such as:
- Difficulty in good surface preparation prior to coating at fabrication and entrapment of welding slag, crevice formation, etc
- Inaccessible regions leading to difficulty in obtaining good coating application, difficulty in inspection and difficult maintenance/re-coating,
- Welds are locations where fatigue damage to the substrate will occur preferentially due to the stress concentration associated with the geometry of the weld toe, leading to cracking of the protective coating
- Joint design may lead to formation of crevices or regions where debris can accumulate leading to more corrosive conditions.
Copper alloying addition to steel has been shown to be beneficial in atmospheric corrosion, forming an adherent patina, e.g. weathering steels such as Cor-Ten, but there are no benefits derived from long term immersion [10,11] and they are not suitable for saline conditions due to the detrimental influence of chloride ions. [3] Further, reference [3] proposes that low alloy steels in atmospheric corrosion may offer some benefit over the carbon steels due to the formation of more dense and tightly adherent corrosion products, which thus will not cause significant undercut of protective coatings. However, it is unknown whether practical advantage has been gained from such an effect.
Corrosion fatigue in oil storage tanks
Where steels are used for the tanks holding sour (hydrogen sulphide containing) oils then there may be concern about corrosion fatigue under such conditions. There are limited data available on corrosion fatigue of materials in sour service, even for the oil and gas sector, which is now generating such information for deepwater riser applications [12,13] for welded pipeline grade steels such as API 5L X65. However, for the shipping industry the concern relates to the handling of crude oil in storage tanks on FPSO and on crude oil tankers, and the performance of welded steelplate used for the hull and storage tanks. Watanabe et al [14] studied the fatigue performance of TMCP steels (yield strengths approx. 415MPa), a carbon manganese steel (yield strength 291MPa) and welded joints in crude oil with 400ppm H 2 S and concluded that acceleration of crack growth rate for the weld occurred in both steels to the same extent compared with air (approximately 7X the rate for parent steels at ΔK ≈30MPa.m -1/2 ) and increasing further as ΔK increases. This is cited as consistent with other steels in similar conditions and indicates conservatively that corrosion fatigue life of steel in sour crude oil may be about 1/10 th that in air, but is not apparently dependent upon the steel. For welded risers, the reduction in fatigue life may be as much as 20X. [13] However, this topic as noted above, is subject to on-going assessment for oil & gas riser applications and the findings may indicate need for generation of similar data for the shipping industry for crude oil cargo tankers and ship-shaped production vessels due to the different fatigue loading regime and temperatures.
Preferential weldment corrosion of carbon steel
Introductory remarks
The influence of welding on the corrosion performance of steels has been studied for many years, reference [15] from 1959 citing earlier work on the subject of preferential corrosion and applying methods of galvanic measurement between weld metal and parent steels. Such corrosion has been reported in a range of different environments from arctic seawater, pipework handling seawater for injection on oil & gas wells, to examples in oil & gas production conditions, mining and chemical processing and has been subject to evaluation for some 40 years. Corrosion has been observed in girth welds and seam welds in pipe and in welds between plates in ships, for many fusion welding processes. As described below, preferential weldment corrosion can be galvanically induced, or related to the microstructure associated with the HAZ and weld metal.
A fact which is frequently overlooked in the case of galvanically driven attack, particularly when engineers may be more familiar with considering the typical galvanic series for selection of different materials which are compatible in dissimilar couples, is that the potential difference between the parent steel and the weldment (weld metal or HAZ being anodic) may only be about 10-15mV. In such cases there is a large uncoated cathodic area of parent steel and a small anodic area (weld metal or HAZ), which then leads to the preferential weldment corrosion. The reason for the influence of surface area is that the cathodic reduction (dissolved oxygen forming hydroxyl ions), which occurs on the parent steel, requires a balancing oxidation at the anode (steel corrosion). To a large extent the efficiency of the cathodic reaction will govern the corrosion rate of the anodic material.
The probable existence of a galvanic couple was noted in early work on HAZ corrosion of welded steel carrying acid mine waters in 1960, [16] but the expectation Robinson noted at the time was that a potential difference of about 100mV was thought to be needed, which subsequently has been shown not to be the case. He attributed the HAZ corrosion to local high residuals tress and hardened microstructure, but later work [17] notes that the cause is more likely microstructure dependent because normalising can eliminate the problem but stress relief did not.
Interestingly, in this latter case referred to by Robinson, [16] the external conditions of constant condensation on the pipework also led to enhanced attack at the welds. TWI does not believe that similar problems have occurred in marine atmospheric conditions. Therefore, this probably reflects the specific conditions of a low pH acidic mine water capable of inducing high corrosion rates and soluble corrosion products, whereas the corrosion products would be more adherent in moist marine conditions.
It is highlighted that preferential weldment corrosion does not imply that the parent steel is galvanically protected by the corroding weldment, but rather, the parent steel is corroding at its intrinsic rate and the weldment corrosion is accelerated above this value. Acceleration of the corrosion rate for steel in seawater service may be between 2-3 times the rate for parent steel alone, hence the need for caution when considering the likelihood o fcorrosion of hull and tanks on shipping based solely on data published for parent steels.
The effects of fusion welding on steels and non-ferrous materials in terms of corrosion behaviour have been summarised in many papers, [17-21] and the key aspects to consider for carbon and low alloy steels are:
- The weld metal is effectively a chill casting due to the rapid cooling rate, unlike the wrought structure of the adjacent parent material, also differing in both composition and inclusion content.
- The parent material experiences temperatures ranging from ambient at some distance from the weld, up to the melting point at the fusion boundary with the weld metal. The consequential metallurgical transformations across the HAZ so formed can significantly affect the intrinsic corrosion rate.
- The thermal history will depend on many interacting factors including the welding heat input, preheat, material thickness, weld bead size and subsequent reheat from additional weld passes.
- The as-deposited hardness of the HAZ will be dependent upon the carbon equivalent of the steel and the heat input, with high heat input processes such as submerged arc welding (SAW) leading to low hardness whilst low heat input with faster cooling rates will result in increased hardness, e.g. manual metal arc (MMA), tungsten inert gas (TIG) and metal inert gas/metal active gas (MIG/MAG) processes. The arc efficiency must be considered when comparing the heat input from different processes.
- The weld metal and HAZ will be covered by an oxide scale, welding slag and flux residue, and the surface of the weld metal will be rough, all of which can affect corrosion behaviour and coating adherence.
- The weld will be a stress raiser in consideration of fatigue or stress corrosion performance due to geometric features such as flaws and the weld profile.
- Due to the thermal expansion and contraction associated with solidification of the weld, residual stresses will remain after welding typically tensile and close to the yield strength in the most severe locations.
It should be noted that the weld metal is invariably selected for the necessary mechanical properties, but where weld metal corrosion is a problem, suitable corrosion resisting consumables can be chosen to overcome the issue whilst retaining joint integrity.
Cases of preferential weldment corrosion in the shipping industry were reported and studied from the 1960s onwards. Originally issues related to the occurrence of attack on ice breakers and vessels operating in icy waters where the paint system would be removed and often the CP system was also compromised. This was still noted to be of concern for shipping in Canadian arctic waters in the 1980s. [22] The subject of preferential weldment corrosion of steel has continued to be studied through the 1960s to the present day, [11, 22-34] both in terms of the specific issues for shipbuilding and more generally to gain understanding of the phenomena. In the past decade however, most research dealing with this issue has related to the occurrence of the problem in the oil & gas sector in sweet production environments. [35]
Preferential weld metal corrosion
The occurrence of preferential weld metal attack in marine environments has been shown to relate to the galvanic difference between the weld metal and the parent steel which can be influenced by the composition of the two materials. However, even where the composition and strength are very similar, accelerated weld metal attack can take place [19] with the flux type affecting the level of acceleration, such that the rate can vary from between 1.5 to 3 times the rate on the parent steel where basic coated electrodes are used. Less acceleration occurs if rutile coated electrodes are used. This was attributed to the inclusion content in the weld metal and whilst rutile coated electrodes performed better, the corrosion rate was still in excess of that of the parent steel. High silicon content has been established as detrimental to weld metal corrosion performance in some process environments but reference [36] determined this was not the case in seawater, and supported the understanding that inclusion content was the controlling factor. The most frequently successful remedy has been selection of welding consumables that have a small alloying content such that the deposited metal will be slightly cathodic to the parent steel, whilst not inducing enhanced HAZ corrosion. Typically electrodes with about 1%Ni or 0.6%Ni plus 0.4%Cu have been found to eradicate the problem in both pipeline steels handling seawater and welded plates for ships hulls.
Fig. 1. Preferential weld metal corrosion in carbon steel
 |
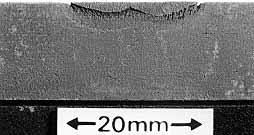 |
|
Fig. 2. Preferential HAZ corrosion in trial bead-on-plate welds in AISI 4130 steel
|
Preferential HAZ attack is not frequently observed, despite the range of microstructures present in the weldment. However, it is believed that the cathodic reduction rate will govern the corrosion rate [18] and that the microstructure will have a secondary role. There is a tendency for preferential HAZ attack to occur in acidic conditions, where the hardened microstructure or the presence of carbides enhance the cathodic reaction of the reduction of hydrogen ions. Therefore, material selection, welding processes and procedures leading to a softer HAZ, through lower carbon and alloy contents and use of high heat input welding parameters will generally result in more uniform corrosion and no enhanced HAZ attack. Avoidance of low temperature transformation products will assist in mitigating such attack. [20]
In addition, the weld thermal cycle can influence the inclusions in the HAZ and this is known to be a problem in electrical resistance welded (ERW) linepipe, where the inclusions can be exposed at the seam weld. Improved chemistry control on the steel source has reduced the occurrence in combination with heat treatment. [19] Despite the long history of assessment of the problem, even today doubt remains over the mechanisms [34] for C-Mn steels, but general agreement exists that avoiding the use of high strength steel with increased Mn is one means of reducing the likelihood of preferential HAZ attack. The work of Brigham et al on steels for arctic waters [22] confirmed that Mn content less than 1% tended to result in no gave preferential HAZ attack, 1.0-1.2% gave some tendency and steels with 1.4%Mn led to HAZ attack. Increased heat input can help to reduce preferential HAZ attack, but it was not possible to correlate to specific microstructures.
It was noted earlier that to avoid preferential HAZ corrosion, high heat inputs during welding are preferred, but it was also stated that to retain the properties of accelerated cooled TMCP steels, low heat input is necessary.Kumakura et al [31] studied the corrosion performance of welds in TMCP steels with strengths in excess of 350MPa in order to demonstrate the acceptability of these materials in shipbuilding applications, recognising the concerns for preferential weldment corrosion performance. The conclusions were that the TMCP parameters could be suitably adjusted to result in bainite microstructures for HAZ and the parent steel, and that welding consumables with controlled Cu and Ni content were necessary to give improved resistance to preferential weldment corrosion compared with a conventional normalised C-Mn ferrite-pearlite steel. Heat input ranges from 2 to 13kJ.mm -1 were assessed for submerged arc welding, (SAW), manual metal arc welding (MMA) and flux cored arc welding (FCAW) by testing for six months in an artificial seawater at 50°C with specimens rotated at3.6-5m.s -1 . The results indicated that the parent steel corrosion rate exceeded that for HAZ or weld metal, thus avoiding preferential corrosion.
The shipbuilding industry is taking advantage of new fabrication technology such as flux cored arc welding (FCAW) and CO 2 laser welding and hybrid laser-arc welding offers further benefits. With any change in welding process, it is particularly important to recognise how it may influence the weld area microstructures and their likely properties. Due to the rapid cooling and absence of reheating for laser and laser-arc hybrid welding, higher HAZ and weld metal hardness may be anticipated compared to conventional arc processes, so a higher likelihood of preferential weld corrosion might be expected. However, low weld metal inclusion levels will affect this effect for the weld metal. It is recommended that the corrosion performance of such weldments be explored in parallel with their mechanical performance, to adequately demonstrate fitness for purpose.
Repair of damage underwater
Damage may be sustained from corrosion, green water impact or collision, and dry docking for repair is costly outside of scheduled maintenance due to loss of revenue. Additionally, for floating production systems, which remain at the field location for many years, the costs associated with unplanned suspension of production and dry docking of a ship-shaped floating production storage and offloading (FPSO) vessel will be significant. Therefore, for fixed structures and FPSOs, long term durability of any repair weld is a requirement, which may also be beneficial to a commercial vessel, which need not then be docked for maintenance until the scheduled date.
Wet underwater repair in the marine environment offers the possibility of repair of ships without need for dry-docking or costly coffer dams etc. With the present requirements for regular maintenance of coatings, the need to repair damage such as areas of high corrosion rate, replacement of preferentially corroded weldments etc. can be scheduled to fit with the planned intervals for commercial shipping. However, if damage occurs necessitating repair, or coatings providing much longer intervals between dry-docking become widely adopted, then wet underwater welding may provide a financially attractive option for repair. Similarly, for ship-shaped FPSOs, the vessel is required to remain on station for many years due to the high downtime costs associated with loss of production, and unplanned shutdown would potentially incur costs between US$0.5-1million per day.
Underwater repair technology has been continuously developed over many years and Nixon provides an overview of the available technologies suitable for repair in the splash zone and shallow water depth for the oil & gas sector, which also have potential application in the shipping industry. [37] As noted by Nixon, wet underwater welding dates back to World War I and is mainly manual metal arc (MMA), but until recently the technique had limitations regarding application to steels in offshore applications due to the riskof hydrogen cracking. Developments such as the use of nickel-based electrodes and temper bead techniques (where multiple weld passes soften the HAZ of the previous deposits) have provided the promise of wider application. The technology is used in the USA, and the AWS Standard AWS D3.6 provides design guidance, but application in the UK North Sea has been restricted to non-critical parts.
Many of the reported applications relate to the repair of structures, typically boat loading platforms, [38] piling supports [39] and offshore platforms. [40] However, there are papers dealing with the application of wet welds for the ship industry allowing vessels to continue in service and thus providing significant benefits to their owners. [41] The objective was to produce high quality wet welds which can be accepted as permanent. Hydroweld have been developing electrodes over a number of years and claim that their product is accepted by the British Royal Navy for permanent repairs to warships. [42] Trials on performance of commercial wet electrodes by TWI and MOD assessed the handle ability from the diver-welder's perspective in combination with assessment of the weld quality. [43] Ongoing work at TWI on the commercialisation of the underwater FCAW process from the Paton Institute in Kiev, Ukraine and other confidential studies provide promise that this approach may be even more widely applicable in future.
However, there are little data published on the corrosion performance of the welds produced by wet underwater welding, or indeed by other underwater techniques. Savich et al report the assessment of high Mn flux-cored wire deposits made underwater. [44] In their judgement, there was a potential difference of 85-90mV between the weld metal and the parent steel but it was claimed that the weld metal was cathodic and therefore not subject to localised corrosion. In view of the possible problems of HAZ attack in high Mn steels, data on long term corrosion performance would be beneficial.
The major concerns regarding corrosion relate to the rapid cooling and thus hardening of an underwater weld HAZ. Therefore it may be expected that preferential corrosion could occur. Also the welds are difficult to produce with good profile and coatings need to be applied below the water-line, all leading to possible concern. Industry will need to demonstrate that such repair welds are not prone to preferential attack over long exposure periods as well as the necessary mechanical integrity, but of course, much benefit can be taken from CP in those cases entirely below the water line.
Corrosion of weldments in corrosion resistant alloys
Introductory comments
Fig. 3. Pitting attack of weld metal in superaustenitic stainless steel (2001-5-1-15-34-57-003)
Corrosion resistant alloys (CRA) include materials such as the austenitic stainless steels, duplex stainless steels, nickel-base alloys and titanium alloys. Their application in shipping is relatively restricted to items such as chemical tanks and associated process pipework. However, for the oil & gas exploration and production sector these alloys are in much wider use due to the need to handle the corrosive fluids produced from the fields and, in the case of titanium alloys for structural weight saving. With increasing use of FPSOs for economic production solutions for marginal fields and the development of deepwater fields, the shipbuilding industry could consider the benefit of developing necessary expertise to allow installation of the process equipment in addition to the construction of the structure/hull.
As for carbon steels, the welding thermal cycle can influence the corrosion performance of weldments in CRA, and therefore due consideration is required at fabrication. In addition vessel owners and operators should be aware of potential areas where the likelihood of corrosion may be higher due to physical configuration and presence of welds, and thus to plan for suitable inspection and maintenance activities.
These alloys will normally be selected for their resistance to the process environment (corrosive gases such as CO 2 and H 2 S, high pressure, high temperature) and may be selected for seawater systems but will obviously be exposed to the external marine environment, and as such consideration must be given to the impact of welding on the corrosion performance. The potentially deleterious impact on behaviour in sour service is beyond the scope of this article, but is widely recognised. In consideration of the behaviour in a chloride-rich atmosphere within the hullor on the topside of ships, the external limitations of the material will usually be dictated by the internal process fluid temperature. CRA whilst usually resistant to general corrosion, are susceptible to localised corrosion such as pitting and crevice attack which can be more prevalent at welds, particularly when the environment is close to the operating envelope of the parent material. They may also be subject to stress corrosion cracking, which is frequently associated preferentially with welds due to residual stress. Hydrogen embrittlement of duplex stainless steels and martensitic stainless steels under CP has been encountered recently and guidance is being developed for offshore pipelines to mitigate against this phenomenon.
Reviews of the influence of welding on corrosion performance of stainless steels and CRA exist [18-21, 45] but in summary the key concerns in modern marine applications are:
- Metallurgical variation across the weldment due to thermal history, different weld metal composition and microstructure due to a preference for non-matching composition consumables, alloy element segregation or formation of intermetallic precipitates in high alloy grades.
- Oxidation leading to depletion in the chromium content in the surface of the HAZ (and weld metal) reducing corrosion resistance.
- Crevice corrosion under oxidation scale or at welding flaws
- Sensitisation to intergranular corrosion
- Residual stress and hence possible increased risk of SCC
- Hydrogen embrittlement under CP.
Stainless steels
Stainless steels gain their resistance to corrosion from the formation of a passive layer of chromium oxide, but this can be susceptible to localised breakdown in the presence of chlorides resulting in pitting corrosion or crevice corrosion, which then propagates as the material cannot reform the passive layer. Additions of molybdenum and nitrogen can enhance resistance to breakdown of the passive film by chloride ions in austenitic stainless steels. A range of alloy compositions have been developed for corrosion resistance (pitting or SCC) and/or improved strength, and thus the service environment must be considered carefully together with likely weldment properties in selecting the material grade. Welding can result in a reduction in corrosion resistance and therefore the welding consumable composition is usually more highly alloyed to mitigate against localised corrosion. In the dual phase duplex stainless steels it is important to maintain the phase balance of ferrite to austenite to around 35-65% in the weldment to ensure adequate corrosion performance in combination with resistance to SCC and necessary toughness for joint integrity.
Stainless steels are frequently compared on the basis of the pitting resistance equivalent number, PREN, an empirical compositional parameter, indicating the benefit of Mo and N as mentioned above, derived from:
PRE=%Cr+3.3%Mo+16%N
Gooch reviewed in detail the factors affecting weldments in stainless steels. [45] Segregation of elements will occur in the weld metal during the rapid cooling of the molten weld pool. Due to this segregation of elements such as chromium and molybdenum the weld metal will have a lower corrosion resistance than the parent material. This is an issue in particular for super austenitic alloys (e.g. 20-25%Cr, 20-25%Ni, 6%Mo, 0.2%N) where the Mo content can decrease to 4% in some parts of the weld metal. Therefore it is common practice to use a nickel-based filler metal with around 9-15%Mo but attack at the unmixed zone at the edge of the weld metal can be experienced at much lower temperatures than expected based on weld metal composition. Intermetallic precipitation can occur in high alloy austenitic stainless steels and duplex stainless steels, leading to a reduction in corrosion resistance. Welding heat input is typically restricted to avoid practical problems.
Sensitisation ('weld decay') due to the formation of chromium carbides at the grain boundaries, particularly in austenitic stainless steels, was a problem several decades ago. The modern steel production methods used for the low carbon 'L' grades have generally reduced the carbon content and thus eliminated this problem. The most likely cause of failure of these alloys in the shipping sector in marine exposure will be pitting and crevice corrosion, and SCC if temperatures are above about 55°C. Locations where salt accumulation can occur, crevices associated with weld flaws and poor paint adherence will be the areas of most concern in the limited application of these alloys in the shipbuilding and shipping sector.
In the shipping sector, CRAs are most likely to be used above the water line and therefore concerns over microbiologically influenced corrosion will not occur. In seawater handling systems, the higher grade alloys can be used and much data exists on their selection and sensitivity to crevice corrosion etc in heat exchanger applications. [46]
Fig. 4. SCC in duplex stainless steel weldment 2000-5-18-10-45-22-003
Austenitic stainless steel AISI 316L has been used for offshore topsides tubing and pipework applications, but due to localised corrosion was subject to replacement by duplex stainless steels (both 22 and 25%Cr grades) and super-austenitic stainless steels (containing 6%Mo). [47] 316L may be a marginal alloy selection in conditions of direct exposure to chlorides from seawater and spray/mist, but the additional costs of nickel base alloys means that for the shipping and offshore sectors austenitic and duplex stainless steels represent the most cost effective material choice. If the materials will be operating at temperatures in excess of about 50°C, 316L will not normally be used without paint protection due to the concerns ofSCC. If the tubing/pipework is insulated then good practice involves painting as there are concerns that moisture ingress to the occluded region will result in corrosion under insulation. Bonding of adhesives and paints to stainlesssteels can be difficult and therefore thermally sprayed aluminium (TSA), could be considered as a means of providing protection at holidays etc, as described later.
In offshore applications, duplex stainless steels and superduplex stainless steels have often been used at temperatures up to about 80°C without concern for coating, although in many circumstances paints will have been applied, particularly for insulated components. However, it appears that there are considerable problems in gaining satisfactory coating performance at elevated temperatures (110-130°C) and thus the O&Gmp;G industry is considering the use of TSA as a means to mitigate against localised corrosion or SCC as discussed later. [48] SCC can propagate very rapidly once initiated and therefore good corrosion mitigation is a pre-requisite for reducing the likelihood of any loss of containment due to pin-holes or cracking.
Titanium
Titanium (commercial purity grade alloys, Grades 1-4) is often favoured in the offshore sector for seawater handling and heat exchanger duties due to its particular resistance to corrosion in seawater. Other alloys are available with Al and V alloying (e.g. Ti6Al4V Grade 5) and Ruthenium or Palladium additions to enhance corrosion performance further if needed, e.g. for high temperature operation. Welding is relatively straight-forward providing excellent inert gas shielding practice is adhered to and thus the alloys are attractive for a long term installation. [49] The material is also likely to retain some value at end of life and on a life cycle cost basis can be accepted over less expensive materials due to the avoidance of maintenance and replacement. There are some concerns over the possibility of sustained load cracking for higher grade alloys which may require further investigation by industry particularly where the alloys are being used for structural and pressure containment applications with long term loading. The phenomena is characterised by failures over extended periods in cases where the stress intensity factor K ISCC is less than the critical value for K lc that necessary for a crack to propagate under impact load.
Corrosion of weldments in aluminium alloys
Aluminium alloys in the 5xxx series of Al-Mg alloys are readily weldable, have good corrosion resistance in marine conditions, but with higher Mg content cold work and ageing can lead to sensitisation to SCC. [50] Alloy 5083 can be used in marine environments without coatings etc provided it is not sensitised to corrosion. The study assessed the performance of welds in alloy 5454 (2.6%Mg) and 5083 (4.6%Mg) produced by MIG welding with consumables of different Mg content and autogenous welds from electron beam welding. The materials were tested for SCC as U-bend specimens immersed in 3%NaCl, including some samples heat treated to sensitise them. Localised corrosion occurred in both MIG welded aluminium alloy grades related to segregation of Mg to the weld toe. Stress corrosion occurred in 5454 sensitised welds made with filler with Mg ≥3.7% but not in sensitised plate, HAZ or weld metals with Mg ≤2.8%, or in any specimens which were not sensitised. Weld metal pitting occurred in all MIG welds in 5454 plate where the Mg content of the weld metal was greater than the 2.6% of the plate material. Using a matching consumable (Mg 4.8%) with 5083 alloy resulted in SCC of sensitised MIG weld metal and HAZ but not the electron beam weld metal or HAZ, nor indeed the unsensitised MIG weld. It was concluded that sensitised 5083 should not be used in conditions likely to lead to stress corrosion and 5454 may be used in welded form if welded autogenously or with a matching filler metal.
In the past decade, significant developments have been made with new welding processes involving friction stir welding (FSW) which was invented by TWI in 1991 and this process is now commercially applied for welding aluminium alloys in the shipbuilding industry and many other sectors. [51] Scandinavian aluminium extruders were the first to commercially apply the process for the manufacture of hollow aluminium panels for deep-freezing of fish and for making panels for ship decks. Friction stir welded structures are now revolutionising the way in which high-speed ferry boats and cruise ships are built from prefabricated lightweight modules. It is believed that no specific problems of corrosion of FSW weldments have been reported in alloys of interest to the shipping sector to date (5xxx and 6xxx series).
There are few data available in the public domain on corrosion of FSW welds in 5xxx and 6xxx series alloys. Frankel and Xia [52] examined welds in 5454 (in O and H34 tempers) and Reynolds [53] also reported on 5454-O. In both instances, they compared results with TIG welds. No appreciable attack was noted in the friction stir welds, which performed as well as or better than TIG welds. Frankel and Xia reported susceptibility to SCC in anodically polarised slow strain rate tests. However, this result should be viewed with some caution as the testing conditions were very severe; Frankel and Xia do point out that SCC tests repeated on U-bend specimens did not reveal any susceptibility. TWI has performed corrosion tests (salt spray and alternate immersion SCC) on 5083-O, as part of a confidential JIP.
There is only one published study on a friction stir welded 6xxx series alloy by Reynolds [53] (alloy 6061-T4). Tests showed that the friction stir weld was less susceptible than its TIG welded counterpart in an intergranular corrosion test. TWI has also performed confidential tests (salt spray) on 6082-T6 in the same JIPas stated above.
Mitigation of corrosion by thermal spraying
Considering the steel structural components and storage tanks in ships, paint systems are required to provide protection against the marine environment and much development is focused upon organic based or silane systems tailored to meet the varying demands from total immersion, splash zone, decking, ullage space, ballast water and crude oil storage etc. For immersed conditions, it is normal practice to incorporate a cathodic protection system as well, either impressed current (external hull) or sacrificial (within storage tanks, or for marine structures/pipelines and risers). Paints act purely as barrier coatings in mitigating marine corrosion; excluding the anti-fouling systems with functional properties for self polishing or controlled release of biocides etc. The need for CP is due to the anticipated degradation of the coating system during operation, which would otherwise allow corrosion of the substrate steel to occur unchecked.
Selection of paint systems to protect the process equipment on oil & gas production topsides and floating production systems, or risers may be inappropriate when operating temperatures are high, or there is need for coating life to match that of the field development. In such cases, the application of TSA may offer the ability to enhance the mitigation provided by a paint barrier system with augmentation by galvanic protection, possibly in combination with cathodic protection for immersed components. The benefits of such coating systems have been demonstrated on civil structures and offshore jackets, and may provide enhanced opportunities to extend the life to first maintenance for coated steel on shipping above the waterline. There is potential also for consideration of use below the waterline and in occluded regions such as storage and ballast tanks, double-hulled tankers etc and in the ullage space. With the likelihood of protection for 20 years, thermally sprayed aluminium may offer the avoidance of need for re-coating the topsides on a vessel with a 25 year design life.
As noted above, the preferential weldment corrosion of carbon steels can be avoided where suitable corrosion protection is applied, inspected and maintained. There are also cases where it may be advantageous to protect corrosion resistant alloys. For all coating systems, the initial surface preparation is critical and this can be made more difficult at welds due to their physical location, geometry and roughened surface profile in contrast with plate material. Coatings which are more tolerant of holidays such as those using thermally sprayed zinc or aluminium, which impart galvanic protection, may be more suited (in combination with a sealant) to application in such areas than paint systems alone.
Thermally sprayed zinc (TSZ) or aluminium can be applied to protect steel, but in acidic or neutral conditions aluminium is favoured. In areas where stainless steels are used, there are concerns about the presence of zinc due to the potential for liquid metal embrittlement in the case of a fire and thus aluminium is again favoured in this respect. Thermally sprayed aluminium offers the benefit of providing local galvanic protection of the substrate steel and has been used in the civil engineering and oil & gas sectors successfully. In the civil sector engineering sector, TSA has been used to protect bridges and for the oil & gas sector it has been applied to offshore structures. Insplash zone and atmospheric exposure, the TSA (and TSZ) allow coatings to remain protective even if the organic sealant is damaged due to abrasion or impact. Immersion allows the coating to protect the substrate where larger holidays are present and the galvanic coupled area can protect the exposed steel. A reduction in consumption rates of sacrificial anodes is also anticipated.
Work by Swidzinski [54] assessed the coating protection of a typical offshore structure. He demonstrated that the initial cost of application for a zinc primer and polysiloxane coating system was £21.50 per square metre (assumed as baseline costi.e. 1), and therefore the relative costs for TSA and sealant system (1.15) was similar, but slightly higher, than that for a four coat zinc epoxy and polyurethane (1.10), whilst the addition of a finish coat to the TSA and sealant raised the cost to a factor of 1.25. However, major cost savings occurred when consideration was given to the total cost for application and the necessary maintenance over 20 years, where a paint system was double the cost of the TSAsystem.
These TSA coatings are damage tolerant and as such can be used on areas subject to impact or wear. Alcan Aluminium produced a variant to enhance the wear and abrasion resistance through incorporation of alumina in the wire, as an aluminium matrix composite, which could be thermally sprayed (Duralcan 90/10). These TSA coatings may provide enhanced protection to superstructure and decking etc.
As noted in the Cost of Corrosion survey for the USA in 2002 [2] , there appears to be a barrier in the shipping sector to accepting the initial higher cost for an improved coating system, driven by change in ownership etc. However, as there are concerns regarding the corrosion within double-hulled tankers, coating systems with additional galvanic capabilities may offer improved confidence in corrosion mitigation.
Presently the oil & gas industry has concerns about the ability of organic paint systems on steel to withstand elevated temperature operation and to last the >35 years needed for a sub sea development. As such, TSA may offer the opportunity for extended coating life and is presently the subject of a Joint Industry Project at TWI [55] to assess performance on steel risers which can operate at temperatures up to about 90°C in the splash zone. In this case, TSA is being considered to extend the coating life to that of the field development which can be up to 50 years.
As noted previously, corrosion resistant alloys such as austenitic and duplex stainless steels can be subject to corrosion problems at welds in the marine environment. In the case of the latter group of 22%Cr and 25%Cr alloys, there is now a concern about the performance of organic paints under insulation on hot process equipment on the topsides of oil & gas production facilities. These alloys can fail due to stress corrosion cracking stress corrosion or pitting and crevice corrosion at elevated temperature in marine operation, perhaps with salt accumulation etc. Stress corrosion cracking propagates rapidly and so it is vital that the coating system prevents moisture contacting the substrate. Paints only offer a barrier protection, and areas of damage may allow localised corrosion/cracking to occur. TWI is assessing the performance of TSA as a system to provide improved mitigation against such attack, whereby the galvanic protection of the aluminium allows the coating to maintain protection even at locations where damage has occurred. [48]
Similarly, the benefit of a TSA or TSZ based coating system with sealant, applied in hot crude oil storage tanks in transport tankers or FPSOs, may allow the coating to last considerably longer. The immersed region at the bottom ofthe tank is problematic as water, debris and heavy oil can accumulate, possibly impacting upon the sacrificial anode performance and the moist ullage space can be an aggressive environment also, particularly due to the heat generated by the crude oil and the presence of acidic condensate. Improved coating performance will allow a reduction in the frequency of maintenance and re-coating, and if the coating is sufficiently robust, it could provide significant cost saving.
Thermally sprayed metallic coatings involving the spraying of corrosion resistant alloys may also be considered. These coatings can include titanium, alloy 625 and similar, but due to the porous nature of the deposited layer requires a sealant to avoid galvanic corrosion of the substrate steel. Presently, it seems unlikely that there will be many applications in the shipping sector for such coatings other than in chemical storage tanks etc.
Conclusions
- Welding typically reduces the corrosion and stress corrosion performance of materials and it is essential that appropriate mitigation measures are employed to weldments intended for marine applications.
- When the corrosion mitigation measures are poor or may fail, such as degradation of coatings with operational life, it is essential that the likelihood of corrosion is reviewed, particularly at weldments and thus the resultant risk of failure is correctly assessed.
- Whilst the underlying principles of preferential weld corrosion are broadly understood, small changes in weld metal chemistry and hardness may be significant, and it is essential that the behaviour of joints produced by improved productivity or new processes are characterised. Particular examples of potential relevance to the marine industry are laser and laser-arc hybrid welding of steels, underwater repairs and friction stir welding of aluminium alloys.
- Thermal spraying technologies providing coatings with improved corrosion mitigation should be assessed for their ability to extend coating life and time to first maintenance for steels and their ability to mitigate localised corrosion and SCC of duplex stainless steels.
References
- Cost of Corrosion (www.corrosioncost.com) US Department of Transport Federal Highway Administration report FHWA-RD-01-156, March 2002.
- Cost of Corrosion, Appendix O - Ships. J T Johnson, in US Department of Transport Federal Highway Administration report FHWA-RD-01-156, March 2002
- Corrosion Vol 1. Ed. L L Shrier, R A Sharman and G T Burstein, Pub. Butterworth Heinemann 1994 pp3:17, 3:18 and 3:29
- H Yajima et al. 'Extensive application of TMCP-manufactured high tensile steel plates to ships hulls and offshore structures', Mitsubishi Heavy Industries Technical Review Vol 24 No1, February 1987.
- K Håkansson 'Personal reflections on 20 years of R&Dmp;D in shipbuilding - its implementation and future aspects'. Proc. Conf. on joining of materials (JOM-8) Helsingor, Denmark 12014 May 1997 pp11-19.
- A guide to the selection of marine materials. INCO databook, 1973
- Y Nakano et al. 'Corrosion fatigue crack propagation behaviour of TMCP steels'. Offshore Mechanics and Arctic Engineering, OMAE 1993. Volume V, Pipeline Technology, pp215-223. Pub. ASME 1993.
- G S Booth, A Maresca and R J Pargeter 'Corrosion fatigue crack growth in a simulated heat affected zone microstructure' TWI Members Report 351/1987
- P Woollin 'Fatigue crack propagation in C-Mn steel HAZ microstructures tested in air and seawater' Offshore Mechanics and Arctic Engineering (OMAE 1998). Proceedings, 17th International Conference, Lisbon, Portugal, 5-9 July 1998, Paper 2601. Ed: C.Guedes-Soares. Publ: New York, NY 10017, USA; ASME International; 1998.
- Corrosion and Corrosion Control: An introduction to corrosion science and engineering. H H Uhlig and R W Revie. pp 175-176, Third edition, Pub: John Wiley & Sons 1985
- G Backman, A Leimalm and S Lundin. Corrosion resistance of high tensile ship steels. Svetsaren English edition, 2-3/71, pp2-11
- TWI Joint Industry Project on Corrosion fatigue of catenary risers in sour service 14135
- J Buitrago and M S Weir 'Experimental fatigue evaluation of deepwater risers in mild sour service', Deep Offshore Technology Conference, New Orleans, USA, November 2002.
- W Watanabe et al, 'Corrosion fatigue strength of ship structural steel plates and their welded joints in sour crude oil'. Offshore Mechanics and Arctic Engineering, OMAE 1994. Volume III, Materials Engineering, pp151-158. Pub. ASME 1994.
- J U MacEwan and H H Yates 'Corrosion of steel weldments', Corrosion Vol15, No1, January 1959, pp18-22
- F P A Robinson 'Mine waters cause grooving corrosion on welded steel piping'. Corrosion, September 1960, pp32-34
- T G Gooch and E N Gregory 'Corrosion aspects of welding structural steel'. British Corrosion Journal 1968, Supplementary Issue, pp48-56
- T G Gooch 'The effect of welding on material corrosion behaviour' NACE International Process Industries Corrosion Seminar, Houston, TX, 13-16 October 1986
- J L Robinson 'Preferential corrosion of welds'. The Welding Institute Research Bulletin Vol. 20, January and February 1979
- D N Noble 'Understanding and preventing corrosion in welded joints' Welding & Metal Fabrication, July 1991, pp293-298
- ASM Handbook Volume 13 Corrosion, pp344-368. ASM International 1987 (5 th printing 1996)
- R J Brigham et al 'Evaluation of weld zone corrosion of shipbuilding steel plates for use in the arctic environment' Canadian Metallurgical Quarterly, 1988 Vol 27 No4 pp311-321
- A Saarinen and K Onnela 'A method for testing the corrodibility of heat affected zones in steel' Corrosion Science 1970, Vol 10, pp809-815
- K Relander and E Tyni 'On the development of Nb- and V-alloyed shipbuilding steels for arctic conditions' The Metallurg Companies Symposium low alloy high strength steels Conf. Nuremburg 21-23 May 1970
- A V A Saarinen 'The effect of mircostructure on the HAZ corrosion of shipbuilding steel' Proc. Int. Conf. The Science and Technology of Iron and Steel (ICSTIS), Supplement Transactions of the Iron and Steel Institute of Japan Vol 11 1971, Section 6 pp1101-1106.
- R Strømmen 'Determination of localized corrosion of heat-affected zones of high tensile C-Mn steels in sea water' International Institute of Welding, Annual Assembly 1975.
- E Räsänen and K Relander 'Development of shipbuilding steel plates for use on arctic seas' Scandinavian Journal of Metallurgy 1978 Vol 7 pp11-17
- W P Gallagher 'Corrosion behaviour of ships' steel plates rolled from continuously-cast slabs' 10 th Offshore Technology Conference, Houston, TX, USA 8-11 May 1978, OTC3191, pp1223-1230.
- K G Leewis 'Weld microstructure and corrosion' Proc Symposium: Welding for a Cold Marine Climate, the New Standard. Halifax, Nova Scotia, Canada 15-16 January 1986, pp32-52
- R J Brigham 'Corrosion of welds in icebreaking ships' Marine Engineering Digest Autumn 1986 pp29-31, Physical Metallurgy Research Laboratories Report PMRL 86-66 (OP-J).
- Y Kumakura et al 'Improved local corrosion resistance of welds of Y.P. 350N/mm 2 class steels for use in icy seas' Proc Conf: 1 st International Offshore and Polar Engineering Conference, Edinburgh, UK, 11-16 August 1991.
- K Itoh et al 'Local corrosion of welds of high-strength steels in seawater' Symposium on corrosion and electrochemistry, 10 October 1985, Halifax, Canada
- G C Savva, G C Weatherly and K T Aust 'Heat-affected zone corrosion behaviour of carbon-manganese steels' Corrosion, March 1989, Vol 45, No3, pp243-249
- M Sephton and P C Pistorius 'Localized corrosion of carbon steel weldments' Corrosion December 2000, Vol 56, No12, pp1272-1279
- J L Dawson et al 'Weld corrosion - chemical, electrochemical and hydrodynamic issues, inconsistencies and models' in Advances in corrosion control and materials in oil and gas production (Proceedings from Eurocorr '97 and Eurocorr '98). European Federation of Corrosion Publications No. 26, Book 715, Pub: Institute of Materials. Ed: P S Jackman and L M Smith, 1999, Chapter 16, pp155-169
- J N Bradley and J C Rowlands 'Corrosion of unprotected and painted steel weldments in seawater' British Welding Journal August 1962, Vol 9, No8, pp476-481
- J H Nixon 'Underwater Repair Technology' Pub: Abington Publishing, Woodhead Publishing Ltd, Cambridge UK, 2000.
- D Keats 'Underwater welding case study', Welding & Metal Fabrication, April 2000, p27
- 'Major wet-welding project undertaken at Mexico port', Offshore, May 1992, pp72-75
- T S Thandavamoorthy, A G Madhava Rao and A R Santhakumar 'Welded repair to damaged offshore platforms - a review', International Welding Conference on Welding and Allied Technology (ICW'99), New Delhi, India, 15-17 February 1999, pp915-922
- M R Johnsen 'Keeping shipshape through underwater welding' Welding Journal November 2001, pp54-57
- M Pett 'Wet (underwater) welding becomes a viable option' Welding & Metal Fabrication, May 1998, pp12-14
- D J Abson and M A Cooper 'Wet underwater welding trial with commercial manual metal arc electrodes' International Seminar: Underwater wet welding and cutting. TWI North, Middlesbrough, UK, 17-18 April 1997 pp50-67
- I M Savich et al 'Prediction of sea-water contact corrosion rate in underwater welded joints of hull steels', in, Welding under extreme conditions. Published on behalf of the International Institute of Welding, Pergamon Press, 1989.
- T G Gooch 'Corrosion behaviour of welded stainless steels': Presented at Comfort A Adams Lecture, AWS 76 th Annual Meeting, Cleveland, OH, USA, 3-7 April 1995. Published: Welding Journal May 1996, pp135s-154s
- European Workshop on 'Sea water corrosion of stainless steels - mechanisms and experiences' European Federation of Corrosion Publications Number 19, Book Number 663, Pub: Institute of Materials 1996.
- A D Batte, A Brown and K Prosser 'The corrosion performance of materials for topsides pipework' Proc: Offshore Mechanics and Arctic Engineering, OMAE 1992, Calgary, Canada, 7-12 June 1992 - Volume III-A, Materials Engineering pp103-112
- TWI Joint Industry Proposal PR6351, 2002 'Thermally Sprayed Aluminium Coatings For Prevention Of Corrosion Of Duplex Stainless Steels At Elevated Temperature'
- Welding Titanium - A designers and users handbook. L S Smith, P L Threadgill and M F Gittos. Titanium Information Group and TWI Ltd, May 1999.
- M F Gittos 'An assessment of the susceptibility of Al-Mg alloy welds to stress corrosion cracking'; TWI Members Report 428/1990
- Second EuroStir ® Workshop 6 November 2002 at TWI, Cambridge UK
- G S Frankel and Z Xia 'Localised corrosion and stress corrosion cracking resistance of friction stir welded aluminium alloy 5454', Corrosion 1999 55 (2), 139-150.
- A P Reynolds 'Mechanical and corrosion performance of TGA and friction stir welded aluminium tailored welded blanks', Proc. Conf. 'Trends in Welding Research - 5 th International Conference', 1-5 June 1998, Mine Mountain, Georgia, USA. 563-567
- M A M Swidzinski 'Offshore Protection: Painting for a 20 year life' Protective Coatings Europe, April 1996, Vol 1, No 4, pp26-40
- TWI Joint Industry Project 'Improving the Reliability and Cost Performance of Thermally Sprayed Aluminium Coatings', 2001-2003, Project Number 13458.